1-2 Principles of Lithium-ion Battery 1-2-1 Mechanism of charge / discharge Lithium-ion batteries do not use metallic lithium, so battery life is not reduced by internal short circuit caused by Lithium dendrites, which was the main weakness of other lithium secondary batteries. Furthermore the chemical stability of carbon anode, even fully charged is higher than metallic lithium. Therefore Lithium-ion batteries are safer systems. Lithium-ion battery consists of lithium cobaltate cathode,graphite anode, organic solvent electrolyte including lithium salt and separator. Fig.1-1 shows schematic for chemical reactions of Lithium-ion batteries. 
Both electrodes have layered structure, andwhen charging, lithium ions come out from the cathode and move through electrolyte to be deposited in between the layers of the graphite anode. When discharging the reaction is revised. Reactions of charge and discharge arefollows. 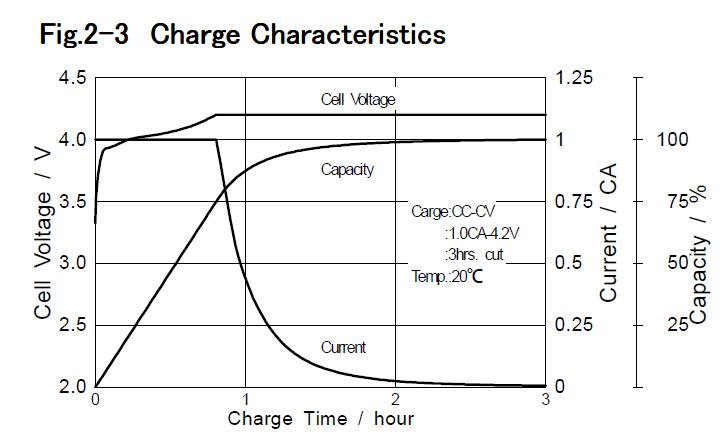
Lithium ions are the medium by whichelectrons are carried and are included in positive electrode materials. Thereis no need to treat metallic lithium in assembling Li-ion cells. During thecharging process, lithium ions in the positive electrode transfer into the negativeelectrode and are placed between the layers of graphite, which makes electricalpotential difference. Since cells are partially charged during the production processfor shipping purposes, the formula shown above can more accurately representedby 1-xCo2/CyLix. 1-2-2 Differences from Nickel Cadmium and Nickel Metal Hydride batteries Ni-Cd and Ni-MH batteries are using alkalineaqueous electrolyte and hydro oxide ions carry the electrical charges. Duringcharging water is created and oxygen gas is generated, so the charge/discharge processloses efficiency to below 100%. However, this sub-reaction can prevent overchargingenabling the manufacture of sealed batteries. Conversely Lithium-ion batteriescharge/discharge process is practically 100% efficient except the first charge/dischargecycle, but this is a disadvantage in overcharge and over-discharge.Lithium-ionbatteries use an organic solvent for the electrolyte which is different fromalkaline rechargeable batteries and the conductivity being lower makes highrate discharge difficult. This is overcome by using larger area electrodes. 1-2-3 Method of manufacturing In order to ensure high performance andsafety,there are many complicated manufacturing processes,and batteries aremade in a carefully controlled environment using strictly controlled andmaintained equipment.The electrodes are manufactured using active materials,conductive agents and binder which are mixed with liquid. These mixtures arethen uniformly coated onto the thin metal foil, then after drying, the electrodesare cut down to the designated sizes. The cathode and anode electrodes are thenwound together with a separator and inserted into a can and the electrolyte isfilled. The sealing completes the battery assembly. Prior to shipping thebatteries to customers, the batteries will undergo an aging process, thorough inspections,initial charge/discharge cycle, etc., ensuring the highest quality of productis maintained. 1-2-4 Cathodes Materials containing lithium ions and whichcan be used the cathode active material must be capable of deintercalation oflithium ions during charge and intercalation of lithium ions during discharge.Lithium cobaltate (LiCoO2) is used mainly in the marketplace and it is knownthat lithium nickelate (LiNiO2) or lithium manganate (LiMn2O4) are also usedfor the cathode. Fig.1-2 shows comparisons of discharge characteristics forvarious cathode materials. Manufactuer is using lithium cobaltate as thecathode because of its good reversibility, capacity, efficiency, voltage andflat discharge characteristics. 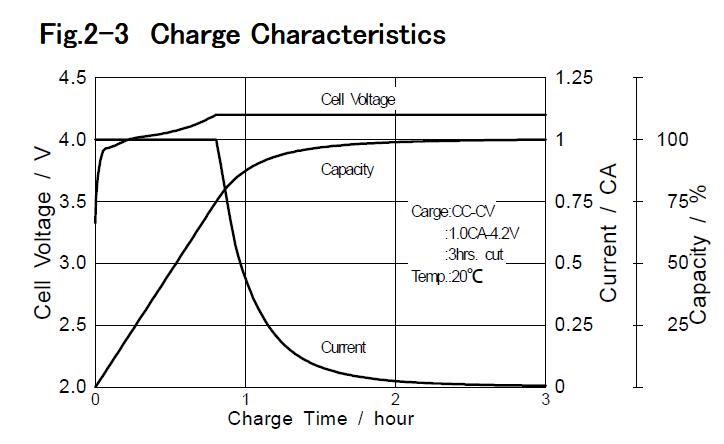
1-2-5 Anodes In order to create Lithium-ion batteries with higher energy densityusing carbon anodes, it must use a carbon material having large lithium storagecapability.Presently, two types of carbon materials are used.One is highlycrystallized carbon like graphite and the other is amorphous carbon like coke.C6Li is equivalent to a lithium ion doped in a hexagonal ring of carbon atoms,and its theoretical capacity is 372mAh/g. Highly crystallized carbon can obtainlarge capacity and graphite can obtain the capacity close to the theoreticalvalue. The biggest difference between coke and graphite is discharge characteristics,and these characteristics are compared in Fig.1-3 Using the coke system, remaining capacity can easily be measuredusing the discharge curve, however graphite system is superior in terms of its fundamentalpurpose of supplying more energy. 1-2-6 Separators Major functions of battery separators are to insulate positive andnegative electrodes, retain the electrolyte and transmit lithium ions. Toensure functionality the separator needs to have the following characteristics; (1) Electrical insulation4.jpg (2) Chemical and thermal stability against electrolyte (3) Capability of holding electrolyte (4) Porous for transmission of lithium ions (5) Thinness and mechanical strength Polyethylene and polypropylene porous thin films are generally usedas suitable materials for abovementioned requirements. The pores of these filmsmelt and prevent the lithium ions from passing through the separator at certaintemperature, which makes a major contribution to safety of Lithium-ion batteriesas instructed in section 4-2. 1-2-7 Electrolyte Electrolyte carries out the essential role of carrying lithium ions(which means current flow).In case of lead acid for Ni-Cd and Ni-MH battery, aqueoussolution is used as electrolyte. However,because Lithium-ion battery is used athigh voltages over 4V, which causes electrolysis of water, lithium salt innonaqueous organic solvent is appropriate for electrolyte. The organic solventis required to satisfy the following characteristics. (1) High conductivity of lithium ion (2) Electric chemical stability (at over 4V) (3) Chemical and thermal stability (4) Wide temperature rage Due to voltages over 4V, only limited typesof solvent are suitable for Lithium-ion batteries. As for electrolyte salt, itis necessary to increase the conductivity of electrolyte and LiPF6 in mixed solventmainly including ethylene carbonate is generally used. 1-2-8 Materials for Can Material for can of positive side have to withstand Lithium-ion’shigh voltage of over 4V. Stainless can be used for 3V, but Lithium-ion battery(cylindrical type) adopts nickel-plated iron for over 4V. | 